Basics of Grain Boundaries in Materials
In crystalline materials, atoms are arranged in a highly ordered repeating pattern called a crystal lattice. However, these materials are rarely a single crystal. Instead, they consist of many small crystals called grains. Each grain has its own crystal orientation, and the regions where grains meet are known as grain boundaries.
Grain boundaries are the interfaces where the crystal orientation changes. They act as distinct zones with different properties compared to the grains themselves. There are several types of grain boundaries, mainly categorized by the angle between the adjoining grains:
- High-angle grain boundaries: These have a large misorientation (typically above 15 degrees). They are more disordered and have higher energy, making them important pathways for processes like diffusion.
- Low-angle grain boundaries: These have a small misorientation (below 15 degrees) and consist of arrays of dislocations. They are less disordered than high-angle boundaries.
- Special boundaries: These include coincident site lattice (CSL) boundaries, which have particularly ordered atomic arrangements and often lower energy and different diffusion characteristics.
The internal structure of grain boundaries is less orderly compared to the grains, with a higher atomic disorder and more free volume. This unique structure allows grain boundaries to serve as faster pathways for atomic movement, or diffusion, compared to the bulk crystal lattice. Because atoms at grain boundaries are less tightly packed and have more defects, they can migrate more easily, making grain boundaries a key feature in understanding material behavior like strength, corrosion resistance, and diffusion rates.
What is Grain Boundary Diffusion
Grain boundary diffusion is the movement of atoms along the boundaries between grains in a crystalline material. Unlike bulk lattice diffusion, where atoms move through the well-ordered crystal structure, grain boundary diffusion happens in the less ordered, more open spaces at the grain edges.
Diffusion is faster along grain boundaries because these boundaries have more defects, extra space, and disturbed atomic arrangements. This creates easier paths for atoms to slip through compared to the tight, regularly spaced atoms inside the grain itself. Think of it like walking through a crowded room (bulk diffusion) versus moving through a wide, empty hallway between rooms (grain boundary diffusion).
This faster atomic movement makes grain boundaries crucial pathways for processes like corrosion, sintering, and material aging. Understanding this difference helps in predicting how materials behave in real-world applications.
Mechanism of Grain Boundary Diffusion
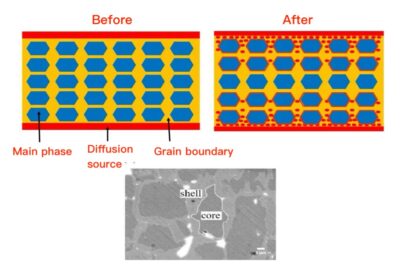
At the atomic level, grain boundary diffusion happens because atoms have more space and less order at the grain boundaries compared to the inside of grains (bulk lattice). This means atoms can jump or move more easily along these boundaries, which act as faster highways for diffusion.
Why Diffusion is Easier at Grain Boundaries
- Atomic Structure: Grain boundaries are regions where the crystal structure is irregular. This disorder creates more open spaces, called free volume.
- Free Volume: Extra spaces between atoms make it easier for atoms to slip through.
- Defect Density: Boundaries contain plenty of defects like dislocations and vacancies that lower the energy barrier for atomic movement.
How It Differs From Bulk Diffusion
| Feature | Grain Boundary Diffusion | Volume (Lattice) Diffusion |
|---|---|---|
| Pathway | Irregular grain boundaries | Well-ordered crystal lattice |
| Atomic Mobility | Higher due to open structure | Lower because atoms are tightly packed |
| Activation Energy | Lower, making diffusion easier | Higher, harder for atoms to move |
| Diffusion Rate | Faster | Slower |
Because of these differences, grain boundary diffusion can dominate at lower temperatures where volume diffusion is limited. Understanding this helps in controlling processes like sintering and corrosion in metals.
Factors Affecting Grain Boundary Diffusion
Several factors influence how fast grain boundary diffusion happens in materials. Temperature plays a big role—higher temperatures give atoms more energy to move, making diffusion quicker. The activation energy for grain boundary diffusion is usually lower than for lattice diffusion, so atoms find it easier to jump along grain boundaries.
Grain size and the type of grain boundaries also matter. Smaller grains mean more grain boundaries, increasing the pathways for diffusion. Likewise, boundaries with different characters—like high-angle versus low-angle—affect diffusion rates due to differences in atomic structure and disorder.
Material purity and composition are important too. Impurities can either block or enhance diffusion depending on their interaction with grain boundaries. Alloying elements might segregate at boundaries, changing diffusion behavior.
Lastly, external stresses impact grain boundary diffusion by altering the atomic spacing or creating defects that can either help or hinder atomic movement. Understanding these factors is key to predicting how materials will behave in real-world conditions.
Measurement and Modeling of Grain Boundary Diffusion
To understand grain boundary diffusion, scientists use specialized techniques that reveal how atoms move along these boundaries. Common methods include:
- Radiotracer techniques: These use radioactive isotopes to track atomic movement over time, providing precise diffusion rates.
- Secondary Ion Mass Spectrometry (SIMS): This method analyzes the composition of surfaces and near-surface regions to map how elements spread along grain boundaries.
Modeling grain boundary diffusion often relies on variations of Fick’s laws, which describe how particles diffuse driven by concentration differences. However, grain boundaries behave differently than bulk materials, so scientists use specific classifications like Harrison’s A, B, and C types:
- Type A: Bulk diffusion dominates; grain boundary diffusion is faster but less significant relative to volume.
- Type B: Both grain boundary and lattice diffusion contribute noticeably.
- Type C: Grain boundary diffusion dominates because lattice diffusion is very slow.
These models help predict how materials will behave under different conditions, such as temperature changes or mechanical stress. This is crucial for designing materials with better durability, especially when grain boundary effects strongly influence processes like corrosion or creep. Overall, measuring and modeling grain boundary diffusion gives us a practical roadmap for improving performance in metals, alloys, and magnetic materials.
Practical Implications and Applications of Grain Boundary Diffusion
Grain boundary diffusion plays a crucial role in many material processes like sintering, creep, corrosion, and embrittlement. Because grain boundaries offer faster atomic pathways compared to the bulk lattice, diffusion along these boundaries can significantly affect how materials behave under heat and stress.
In sintering, grain boundary diffusion helps particles fuse together more efficiently, improving density and mechanical strength. During creep—where materials slowly deform under constant stress—grain boundary diffusion enables atoms to move more easily, influencing long-term durability. However, in corrosion and embrittlement, this faster diffusion along grain boundaries can lead to weak spots, making materials more vulnerable to failure.
For magnetic materials, especially those manufactured at NBAEM, controlling grain boundary diffusion is essential. It directly impacts magnetic properties by affecting grain structure and purity. Managing diffusion helps enhance magnetic performance, mechanical strength, and the overall lifespan of magnets. This is particularly important in high-performance magnetic materials where stability and durability are key.
By understanding and optimizing grain boundary diffusion, NBAEM ensures its magnets maintain excellent quality, combining strong magnetic performance with mechanical resilience. This knowledge supports innovations in material design that meet the demanding needs of the U.S. market for reliable, high-quality magnetic components. For more insight into magnetic materials, see What Is High-Performance SmCo Magnets and What Is Permanent Magnet.
Grain Boundary Diffusion in Magnetic Materials
Grain boundary diffusion plays a unique role in magnetic materials, impacting their magnetic domains and overall performance. Unlike bulk diffusion, movement along grain boundaries can alter the arrangement of atoms and magnetic domain walls more rapidly. This can either enhance or degrade magnetic properties depending on the material and processing conditions.
One challenge is that excessive grain boundary diffusion can lead to unwanted changes in magnetic alignment, causing reduced coercivity or magnetization. On the flip side, controlled diffusion at grain boundaries can improve the uniformity of magnetic domains, boosting the stability and strength of magnets.
For example, in rare-earth magnets like SmCo and NdFeB, managing grain boundary diffusion helps maintain a fine grain structure, which is critical for high magnetic performance and thermal stability. This is essential in applications requiring strong, reliable magnets, such as electric motors or data storage devices.
Understanding and controlling grain boundary diffusion also aids in minimizing magnetic aging and enhancing resistance to corrosion and embrittlement, common problems in the magnetic materials industry. These advantages make grain boundary diffusion a key factor in producing high-performance magnets tailored for demanding US markets.
To learn more about the basics of magnets and magnetic poles, check out what is a rare-earth magnet and what are magnetic poles.



Leave A Comment